Research Areas and Ongoing Projects
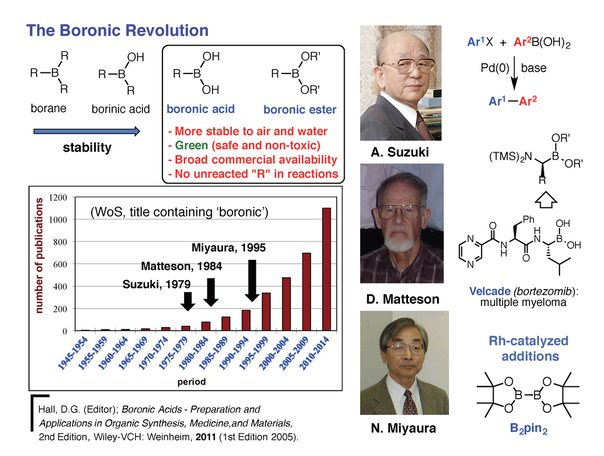
Modern organic synthesis is a powerful discipline that defines humankind’s capacity to create and transform new molecules with tailored properties and functions that can improve our standard of living. With inventions like antibiotics and other pharmaceutical drugs, it can be stated that organic synthesis is largely responsible for the significant increase in life expectancy observed in modern societies over the past century. To further advance, organic synthesis relies on the development of new reaction methods that enable the efficient construction of compounds with precise structural characteristics. The Hall Laboratory is active in several areas of organic synthesis with a focus on the applications of organoboronic acids, a modern research topic that has been in continuous expansion in the past two decades. Although they are historically recognized mostly for their use as reagents in the Nobel-prize winning Suzuki-Miyaura cross-coupling reaction, boronic acids and their esters have emerged into a versatile class of “jack-of-all-trades” compounds with multiple applications ranging from catalysis to medicine (Figure 1). [1]
Modern organic synthesis is a powerful discipline that defines humankind’s capacity to create and transform new molecules with tailored properties and functions that can improve our standard of living. With inventions like antibiotics and other pharmaceutical drugs, it can be stated that organic synthesis is largely responsible for the significant increase in life expectancy observed in modern societies over the past century. To further advance, organic synthesis relies on the development of new reaction methods that enable the efficient construction of compounds with precise structural characteristics. The Hall Laboratory is active in several areas of organic synthesis with a focus on the applications of organoboronic acids, a modern research topic that has been in continuous expansion in the past two decades. Although they are historically recognized mostly for their use as reagents in the Nobel-prize winning Suzuki-Miyaura cross-coupling reaction, boronic acids and their esters have emerged into a versatile class of “jack-of-all-trades” compounds with multiple applications ranging from catalysis to medicine (Figure 1). [1]
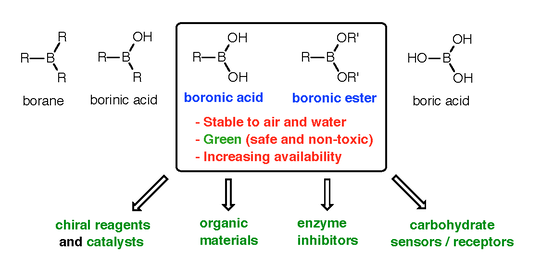
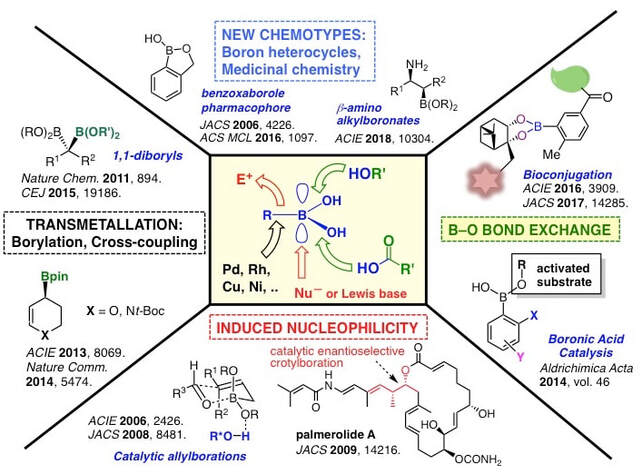
Examples of our lab's past contributions include stereocontrolled synthetic methodologies such as catalytic enantioselective allylborations [2,3] and multicomponent reactions [4], double-allylation reagents [5], organocatalysis with arylboronic acids [6], the development of resins and linkers for the solid-phase synthesis of boronic acids [7,8], natural product synthesis [9-12], oligoboronic acid receptors for complex oligosaccharides [13,14], and 'click' bioconjugation [15-17]. Details of all these contributions can be found in our Publication Section. Our overarching goal is to develop new reactions and strategies to access functional molecules with potential applications in biology and medicine. We pursue these objectives by exploiting the many modes of reactivity accessible to boronic acids (Figure 2).
Students involved in these research projects are exposed to a multidisciplinary environment that provides ample opportunity to develop expertise in a wide range of modern concepts and techniques of organic synthesis and chemical biology. The following sections summarize ongoing and future research projects potentially available to students interested in joining our group:
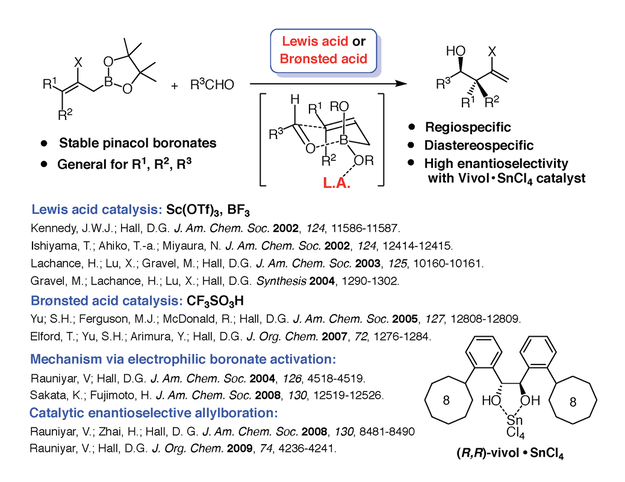
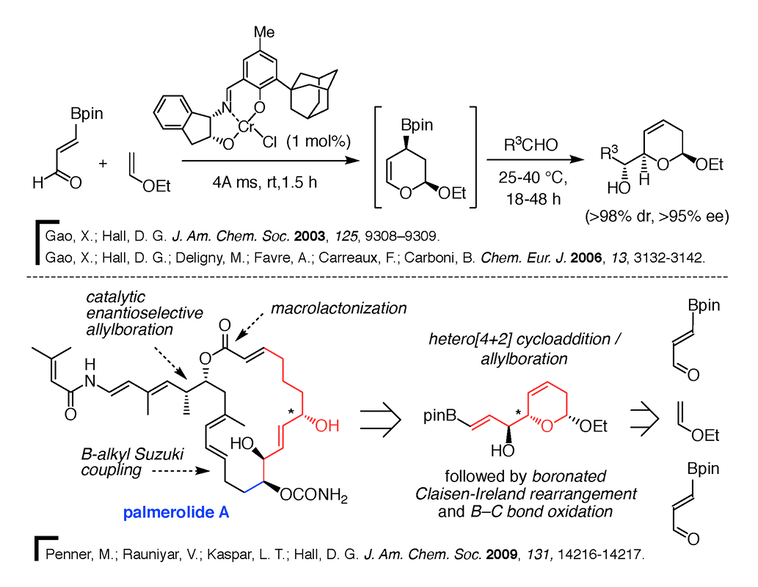
1) Synthetic methodology and stereocontrol using organoboron chemistry. Organoboron compounds are extremely useful as stable reagents, intermediates, and catalysts in organic synthesis. Several applications of these compounds remain to be discovered, and our group is particularly interested in developing powerful asymmetric carbon-carbon bond forming methods based on boronic acids and their esters. The ability to selectively synthesize one of two enantiomeric forms of a molecule is very important in the pharmaceutical industry. One such reaction is the catalytic enantioselective allylboration of aldehydes optimized in our group (Figure 3) [3]. This method makes use of diol-metal complexes as chiral Brønsted acids. The optimal diol we identified, Vivol, was nicknamed after Vivek Rauniyar, the graduate student from our group who discovered it.
1) Synthetic methodology and stereocontrol using organoboron chemistry. Organoboron compounds are extremely useful as stable reagents, intermediates, and catalysts in organic synthesis. Several applications of these compounds remain to be discovered, and our group is particularly interested in developing powerful asymmetric carbon-carbon bond forming methods based on boronic acids and their esters. The ability to selectively synthesize one of two enantiomeric forms of a molecule is very important in the pharmaceutical industry. One such reaction is the catalytic enantioselective allylboration of aldehydes optimized in our group (Figure 3) [3]. This method makes use of diol-metal complexes as chiral Brønsted acids. The optimal diol we identified, Vivol, was nicknamed after Vivek Rauniyar, the graduate student from our group who discovered it.
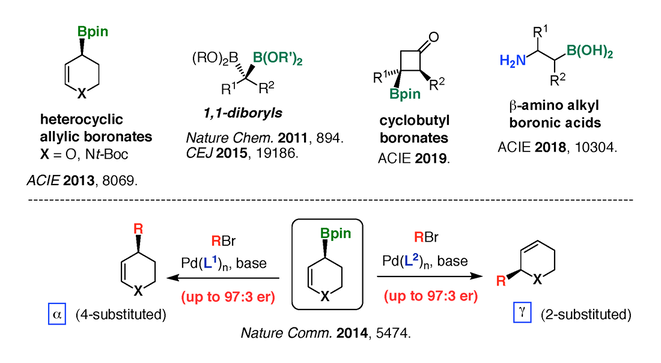
More recently, our work in stereoselective synthesis has been focusing on the preparation and use of highly functionalized, pharmaceutically relevant chiral boronates such as heterocyclic and cyclobutyl derivatives, as well as beta-amino alkylboronates (Figure 4). Heterocyclic allylic boronates can be employed in carbonyl allylation18,19 and stereospecific cross-coupling [20]. Stereoselective preparation and cross-coupling of gem-diboryl alkanes provides an efficient route to biologically relevant diarylalkane units [21]. In a collaboration with a team of medicinal chemists at Pfizer, we optimize various methods to produce chiral cyclobutylboronates [22], which are attractive intermediates in the preparation of natural products and pharmaceutical drugs. beta-Aminoalkylboronic acids are biologically relevant bioisosteres of beta-aminoacids. Because there is a lack of effective and general methods to prepare these compounds, we address this problem by developing stereodivergent methods to access all stereosiomers of beta-aminoalkylboronates [23].
2) Multicomponent reactions in natural product synthesis. Multicomponent reactions are broadly defined as reactions that make use of three or more substrates simultaneously or sequentially without the need for changing the solvent. These atom-economical processes are particularly attractive in combinatorial chemistry, drug discovery, and even in natural product synthesis. In this context, our group has developed a tandem three-component [4+2] cycloaddition/allylboration strategy to assemble a-hydroxyalkyl piperidine derivatives parent to polyketides, piperidine alkaloids and azasugar analogues [4]. A catalytic enantioselective variant has also been developed for making pyrans [4], and this multicomponent reaction was employed in the total syntheses of the potent antibiotic thiomarinol H10 and the novel anticancer agent palmerolide A11 (Figure 5). Palmerolide A was isolated from a tunicate in the Antarctic Ocean, and is a potent antimelanoma agent with great potential for the treatment of certain types of skin cancers.
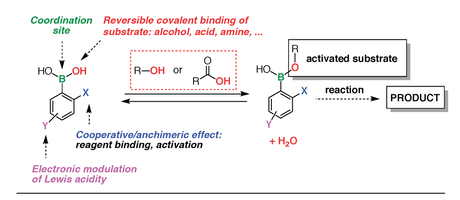 Figure 6. Concept of bifunctional organocatalysis with boronic acids.
Figure 6. Concept of bifunctional organocatalysis with boronic acids.
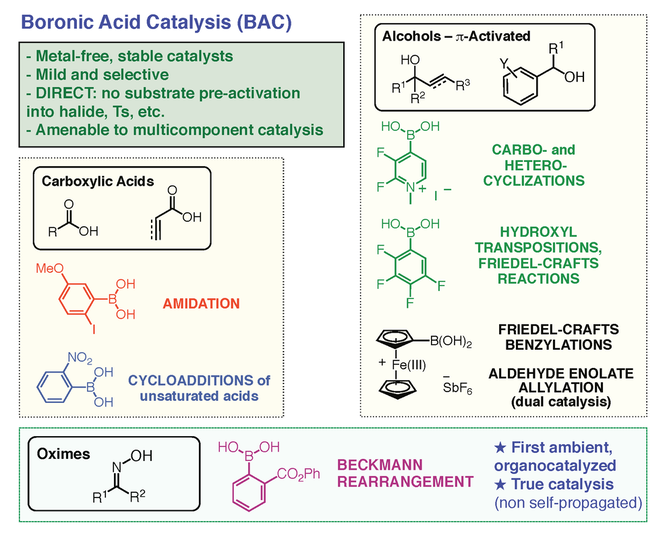
3) Boronic Acid Catalysis (BAC): greener activation of hydroxy compounds with boronic acids. The role of boronic acids as catalysts has long been neglected. These cool compounds have the unique capability of exchanging with alcohols and carboxylic acids reversibly, and this property can be put to use in catalysis to achieve temporary activation and/or templating effects (Figure 6).
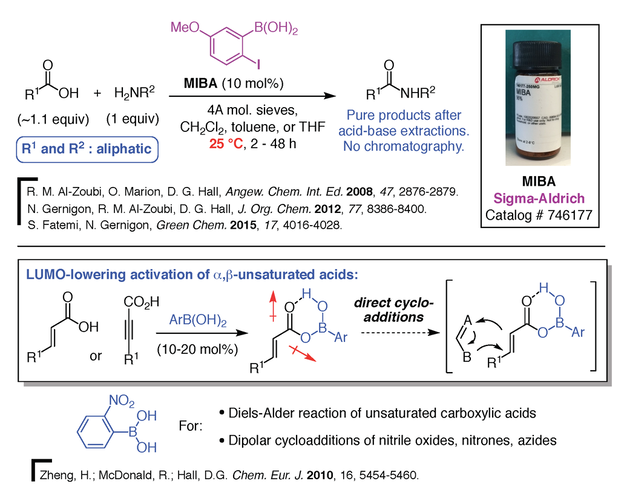
We have exploited this potential with the surprising and impressive discovery that ortho-haloboronic acids such as ortho-iodophenylboronic acids (MIBA catalyst) can catalyze direct amidation reactions between free carboxylic acids and amines at room temperature (Figure 7) [6,24]. This discovery has great practical implications because amides are one of the most important functional groups in Nature and in pharmaceuticals. In Nature, they are ubiquitous as the key units to the backbone of peptides and proteins. Unfortunately, traditional methods for the preparation of amides continues to challenge chemists and there are still no means to make amides in a simple, green and atom-economical fashion. In contrast, amidations catalyzed by ortho-iodophenylboronic acid do not create any waste, giving water as the only by-product, and the amide products can be isolated using just simple acid-base extractions. The direct activation of carboxylic acids can also be applied to Diels-Alder reactions and dipolar cycloadditions of unsaturated carboxylic acids (Figure 7) [25]. Our laboratory also designed a series of electrophilic boronic acids catalysts for direct cyclizations and Friedels-Crafts-type alkylations of allylic and benzylic alcohols [26], as well as the activation of ketoximes in the Beckmann rearrangement (Figure 8) [27]. Two of our catalysts, MIBA, for direct amidations, and the Beckmann rearrangement catalyst, are commercialized by Sigma-Aldrich.
The preparation of chiral arylboronic acids could lead to stereoselective applications of these reactions. Furthermore, other ongoing directions in BAC include dual catalysis combining boronic acids and light, as well as other modes of promoting chemical reactions.
We have exploited this potential with the surprising and impressive discovery that ortho-haloboronic acids such as ortho-iodophenylboronic acids (MIBA catalyst) can catalyze direct amidation reactions between free carboxylic acids and amines at room temperature (Figure 7) [6,24]. This discovery has great practical implications because amides are one of the most important functional groups in Nature and in pharmaceuticals. In Nature, they are ubiquitous as the key units to the backbone of peptides and proteins. Unfortunately, traditional methods for the preparation of amides continues to challenge chemists and there are still no means to make amides in a simple, green and atom-economical fashion. In contrast, amidations catalyzed by ortho-iodophenylboronic acid do not create any waste, giving water as the only by-product, and the amide products can be isolated using just simple acid-base extractions. The direct activation of carboxylic acids can also be applied to Diels-Alder reactions and dipolar cycloadditions of unsaturated carboxylic acids (Figure 7) [25]. Our laboratory also designed a series of electrophilic boronic acids catalysts for direct cyclizations and Friedels-Crafts-type alkylations of allylic and benzylic alcohols [26], as well as the activation of ketoximes in the Beckmann rearrangement (Figure 8) [27]. Two of our catalysts, MIBA, for direct amidations, and the Beckmann rearrangement catalyst, are commercialized by Sigma-Aldrich.
The preparation of chiral arylboronic acids could lead to stereoselective applications of these reactions. Furthermore, other ongoing directions in BAC include dual catalysis combining boronic acids and light, as well as other modes of promoting chemical reactions.
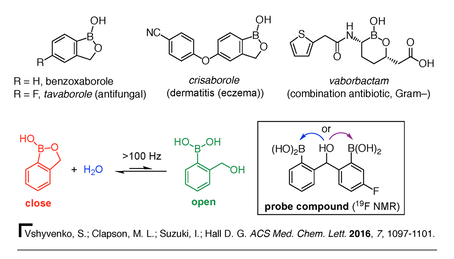
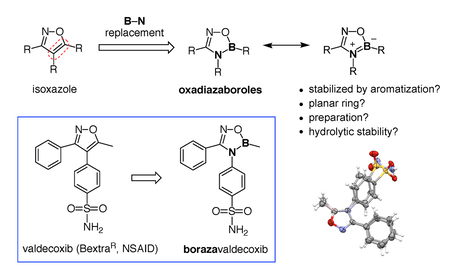
4) Boron heterocycles. Once viewed as a chemical curiosity, boron-containing heterocycles are now emerging as a remarkable class of compounds with great utility. For example, in the past decade, the benzoxaborole ring gained acceptance as a new pharmacophore (i.e., a bioactive part or group of atoms in a molecule). This progress is strongly supported by the recent approval of the antifungal drug Tavaborole and the $5.5 billion USD sale of Anacor Pharmaceuticals to Pfizer for the rights to Crisaborole (EucrisaTM), a drug projected to vastly improve the standard of care for eczema and psoriasis (Figure 9). Our team made seminal contributions on the reactivity and properties of benzoxaboroles, such as their special ability to complex carbohydrates [28-29], and their dynamic hydrolysis [30]. In a collaboration with Prof. Ravin Narain (U of A Chemical and Materials Engineering), we design benzoxaborole-containing self-healing responsive nanogels for biomedical applications [31]. As new and stable chemotypes, boron-containing rings present a new landscape for drug discovery and other applications such as catalysis and materials science. Our laboratory has initiated a program on the design and study of new boron heterocycles that can open new opportunities for fundamental breakthroughs and translational activities of potential benefit to society. For example, we recently reported on the bioisosterism of isozaxole drugs where a C=C bond can be replaced by a geometrically and electronically equivalent B–N bond (Figure 9, bottom) [32]. Ongoing projects focus on the design, synthesis, and evaluation of the reactivity and physical properties of novel aromatic and non-aromatic boron heterocycles. These new compounds are then evaluated for their potential as anti-infective agents in collaboration with the Community for Open Antimicrobial Drug Discovery (CO-ADD) and other organizations.
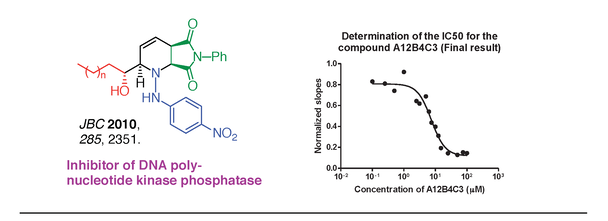
5) Medicinal chemistry and chemical biology. Our group is involved in a number of medicinal chemistry collaborations, especially in the area of oncology. For example, we have exploited a boron-based multicomponent reaction developed in our laboratory to synthesize parallel libraries of drug-like imidopiperidine compounds [33]. In collaboration with the lab of Prof. Michael Weinfeld (Cross Cancer Institute, UofA Oncology), these compounds were found to be potent inhibitors of the human polynucleotide kinase/phosphatase (PNKP) [34]. PNKP is a DNA repair enzyme involved in the repair of strand breaks. It possesses two activities; a DNA 3'-phosphatase and a 5'-kinase. Cancer cells depleted of hPNKP display an increased sensitivity to ionizing radiation and certain chemotherapeutic agents, thus inhibition of hPNKP could increase the efficacy of cancer treatment. As part of a large multidisciplinary collaboration, we design and synthesize derivatives of the initial hit, A12B4C3, for structure-activity relationships studies with the goal of developing a lead inhibitor suitable for clinical applications.
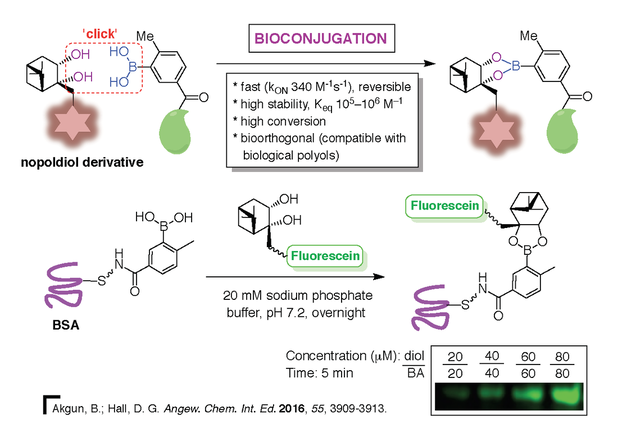
The ability of boronic acids to complex polyols in aqueous media can be of great use in chemical biology. Recently, we have explored the application of boronic ester formation in 'click' bioconjugation (Figure 10, bottom) [15]. Boronic ester formation has proven to provide tight-binding conjugates that are bioorthogonal. A second-generation system was tested in cell tagging [16] and studies are ongoing to evaluate this system in live imaging.
The ability of boronic acids to complex polyols in aqueous media can be of great use in chemical biology. Recently, we have explored the application of boronic ester formation in 'click' bioconjugation (Figure 10, bottom) [15]. Boronic ester formation has proven to provide tight-binding conjugates that are bioorthogonal. A second-generation system was tested in cell tagging [16] and studies are ongoing to evaluate this system in live imaging.
References
1) D. G. Hall, Editor; Boronic Acids – Preparation and Applications in Organic Synthesis, Medicine, and Materials; Wiley-VCH, 2nd Edition, 2011. [Book]
2) D. G. Hall; Lewis and Brønsted Acid-Catalyzed Allylboration of Carbonyl Compounds: from Discovery to Mechanism and Applications; Synlett 2007, 1644-1655. [Publication 47]
3) V. Rauniyar, H. Zhai, D. G. Hall; Catalytic Enantioselective Allyl- and Crotylboration of Aldehydes Using Chiral Diol-SnCl4 Complexes. Optimization, Substrate Scope and Mechanism Investigations; J. Am. Chem. Soc. 2008, 130, 8481-8490. [Publication 55]
4) D. G. Hall, T. Rybak, T. Verdelet. Multicomponent Hetero-[4 + 2] Cycloaddition/Allylboration Reaction: From Natural Product Synthesis to Drug Discovery, Acc. Chem. Res. 2016, 49, 2489-2500.
5) F. Peng, D. G. Hall; Simple, Stable and Versatile Double-Allylation Reagents for the Preparation of Skeletally Diverse Compounds; J. Am. Chem. Soc. 2007, 129, 3070-3071. [Publication 46]
6) Review: D. G. Hall. Boronic Acid Catalysis, Chem. Soc. Rev. 2019, 48, 3475-3496.
7) D. G. Hall, J. Tailor, M. Gravel; N,N-Diethanolaminomethyl Polystyrene: An Efficient Resin to Immobilize Boronic Acids; Angewandte Chemie Int. Ed. 1999, 38, 3064-3067. [Publication 2]
8) M. Gravel, K. A. Thompson, M. Zak, C. Berube, D. G. Hall; Universal Solid-Phase Approach to the Immobilization, Derivatization, and Resin-to-Resin Transfer Reactions of Boronic Acids; J. Org. Chem. 2002, 67, 3-15. [This article was featured in the cover page graphics of Issue 1, 2002] [Publication 11]
9) S. H. Yu, M.J. Ferguson, R. McDonald, D. G. Hall; Brønsted Acid-Catalyzed Allylboration: Short and Stereodivergent Synthesis of All Four Eupomatilone Diastereomers with Crystallographic Assignments; J. Am. Chem. Soc. 2005, 127, 12808-12809. [Publication 36]
10) X. Gao, D. G. Hall; Catalytic Asymmetric Synthesis of a Potent Thiomarinol Antibiotic, J. Am. Chem. Soc. 2005, 127, 1628-1629. [Publication 32]
11) M. N. Penner, V. Rauniyar, L. Kaspar, D. G. Hall; Catalytic Asymmetric Synthesis of Palmerolide A via Organoboron Methodology, J. Am. Chem. Soc. 2009, 131, 14216-14217.
12) T. E. Elford, D. G. Hall; Total Synthesis of (+)-Chinensiolide B via Tandem Allylboration/Lactonization, J. Am. Chem. Soc. 2010, 132, 1488-1489.
13) S. Manku, D. G. Hall; Synthesis, Decoding, and Preliminary Screening of a Bead-Supported Library of Triboronic Acid Receptors for Complex Oligosaccharides; Australian Journal of Chemistry 2007, 60, 824-828. [Publication 51]
14) A. Pal, M. Bérubé, D. G. Hall; Design, Synthesis, and Screening of a Library of Peptidyl Bis-Boroxoles as Low Molecular Weight Receptors for Complex Oligosaccharides in Water: Identification of a Receptor for the Tumour Marker TF-Antigen, Angew. Chem. Int. Ed. 2010, 49, 1492-1495.
15) B. Akgun, D. G. Hall. Fast and Tight Boronate Formation for ‘Click’ Bioorthogonal Conjugation, Angew. Chem. Int. Ed. 2016, 55, 3909-3913.
16) B. Akgun, C. Li, Y. Hao, G. Lambkin, R. Derda, D. G. Hall. Synergic ‘Click’ Boronate/Thiosemicarbazone System for Fast and Irreversible Bioorthogonal Conjugation in Live Cells, J. Am. Chem. Soc. 2017, 139, 14285-14291.
17) Review: B. Akgun, D. G. Hall. Boronic Acids as Bioorthogonal Probes in Site-Selective Labeling of Proteins, Angew. Chem. Int. Ed. 2018, 57, 13028-13044.
18) S. Lessard, F. Peng, D. G. Hall; Alpha-Hydroxyalkyl Heterocycles via Chiral Allylic Boronates: Pd-Catalyzed Borylation Leading to a Formal Catalytic Enantioselective Isomerization of Allylic Ether/Amine, J. Am. Chem. Soc. 2009, 131, 9612-9613.
19) J. Ding, D. G Hall, Concise Synthesis and Antimalarial Activity of All Four Mefloquine Stereoisomers Using a Highly Enantioselective Catalytic Borylative Alkene Isomerization, Angew. Chem. Int. Ed. 2013, 52, 8069-8073.
20) J. Ding, T. Rybak, D. G. Hall. Synthesis of Chiral Heterocycles Selectivity by Ligand-Controlled Regiodivergent and Enantiospecific Suzuki-Miyaura Cross-Coupling. Nature Communications 2014, 5, 5474-5482.
21) J. C. H. Lee, R. McDonald, D. G. Hall; Enantioselective Preparation and Chemoselective Cross–Coupling of 1,1-Diboron Compounds, Nature Chemistry 2011, 3, 894-899.
22) H. A. Clement, M. Boghi, R. M. McDonald, L. Bernier, J. W. Coe, W. Farrell, C. J. Helal, M. R. Reese, N. W. Sach, J. C. Lee, D. G. Hall, High-Throughput Ligand Screening Enables the Enantioselective Conjugate Borylation of Cyclobutenones to Access Synthetically Versatile Tertiary Cyclobutylboronates, Angew. Chem. Int. Ed. 2019, in press.
23) X. Li, D. G. Hall. Diastereocontrolled Protodeboronation of β-Sulfinimido gem-Bis(boronates): A General and Stereoselective Access to α,β-Disubstituted β-Aminoalkylboronates, Angew. Chem. Int. Ed. 2018, 57, 10304-10308.
24) N. Gernigon, R. Al-Zoubi, D. G. Hall; Direct Amidation of Carboxylic Acids Catalyzed by ortho-Iodo Arylboronic Acids: Catalyst Optimization, Scope, and Preliminary Mechanistic Study Supporting a Peculiar Halogen Acceleration Effect, J. Org. Chem. 2012, 77, 8386-8400. {2012 Journal of Organic Chemistry Outstanding Paper of the Year Award}
25) H. Zheng, D. G. Hall; Boronic Acid Catalysis for Mild and Selective [3+2] Dipolar Cycloadditions to Unsaturated Carboxylic Acids, Chem. Eur. J. 2010, 16, 5454-5460.
26) X. Mo, J. Yakiwchuk, J. Dansereau, J. A. McCubbin, D. G. Hall. Unsymmetrical Diarylmethanes by Ferroceniumboronic Acid Catalyzed Direct Friedel-Crafts Reactions with Deactivated Benzylic Alcohols: Enhanced Reactivity due to Ion-Pairing Effects, J. Am. Chem. Soc. 2015, 137, 9694-9703.
27) X. Mo, T. D. R. Morgan, H. T. Ang, D. G. Hall. Scope and Mechanism of a True Organocatalytic Beckmann Rearrangement with a Boronic Acid / Perfluoropinacol System Under Ambient Conditions, J. Am. Chem. Soc. 2018, 140, 5264-5271.
28) M. Dowlut, D. G. Hall; An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water; J. Am. Chem. Soc. 2006, 128, 4226-4227. [Publication 41]
29) M. Bérubé, M. Dowlut, D. G. Hall; Benzoboroxoles as Efficient Glycopyranoside-Binding Agents in Physiological Conditions: Structure and Selectivity of Complex Formation; J. Org. Chem. 2008, 73, 6471-6479. [Selected as a “Feature Article” and for Cover Page] [Publication 57]
30) S. Vshyvenko, M. Clapson, I. Suzuki, D.G. Hall. Characterization of the Dynamic Equilibrium Between Close and Open Forms of the Benzoxaborole Pharmacophore. ACS Med. Chem. Lett. 2016, 12, 1097-1101.
31) D. Wu, W. D. Wang, D. Diaz-Dussan, Y. Y. Peng, Y. J. Chen, R. Narain, D. G. Hall. In Situ Forming, Dual-Crosslink Network, Self-Healing Hydrogel Enabled by a Bioorthogonal Nopoldiol-Benzoxaborolate Click Reaction with a Wide pH Range, Chemistry of Materials 2019, 31, 4092-4102.
32) M. Boghi, D. G. Hall. Valdecoxib vs Borazavaldecoxib: Isoxazole B=N Isosterism as a Case Study in Designing and Stabilizing Boron Heterocycles, Org. Biomol. Chem. 2018, 16, 4849-4856.
33) A. Ulaczyk-Lesanko, E. Pelletier, M. Lee, H. Prinz, H. Waldmann, D. G. Hall; Optimization of Three- and Four-Component Reactions for Polysubstituted Piperidines. Application to the Synthesis and Preliminary Biological Screening of a Prototype Library, Journal of Combinatorial Chemistry 2007, 9, 695-703. [Publication 50]
34) G. K. Freschauf, R. S. Mani, T. R. Mereniuk, M. Fanta, C. A. Virgen, G. L. Dianov, J.-M. Grassot, D. G. Hall, M. Weinfeld; Mechanism of Action of an Imido-piperidine Inhibitor of Human Polynucleotide Kinase/Phosphatase, J. Biol. Chem 2010, 285, 2351-2360.