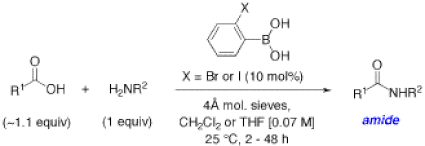Our research interests are very diverse, although there is a significant focus on synthetic organoboron methodology with applications in natural products, catalysis, chemical biology, and medicinal chemistry. This multidisciplinary environment provides students with the opportunity to develop expertise in a wide range of contemporary techniques that show increasing importance in biotechnology and pharmaceutical research.
1) Introduction to organoboron compounds
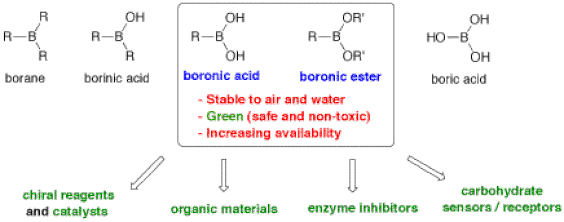
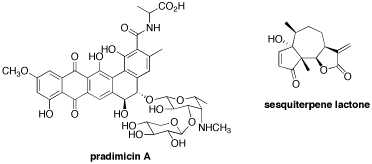
Modern organic synthesis is a powerful discipline that defines humankind’s capacity to create and transform new molecules with tailored properties and functions that can impact our standard of living. As such, it is said that organic synthesis is largely responsible for the significant increase in life expectancy observed in modern societies over the past century. To further advance, organic synthesis relies on the development of new reaction methods that enable the efficient construction of compounds with precise structural characteristics. The Hall Laboratory is active in several areas of organic synthesis with a focus on the applications of organoboronic acids. Boronic acids and their esters are versatile “jack-of-all-trades” compounds with multiple applications ranging from catalysis to medicine (Figure 1). 1
Examples of our recent contributions include stereocontrolled synthetic methodologies such as catalytic enantioselective allylborations2,3 and multicomponent reactions,4,5 double-allylation reagents,6 organocatalysis with arylboronic acids,7 the development of resins and linkers for the solid-phase synthesis of boronic acids,8,9 natural product synthesis,10-12 and the synthesis and characterization of bead-supported libraries of polyamine libraries and oligoboronic acid receptors for the selective recognition of complex oligosaccharides.13 Details of all these contributions can be found in our Publication Section. Our global research objective is to develop new reactions and strategies to access functional molecules with potential applications in biology and medicine. Students involved in these research projects are exposed to a multidisciplinary environment that provides opportunity to develop expertise in a wide range of modern concepts and techniques of organic synthesis, combinatorial chemistry, and chemical biology. The following sections summarize ongoing and future research projects potentially available to any students interested in joining our group.
2) Synthetic methodology and stereocontrol using organoboron chemistry
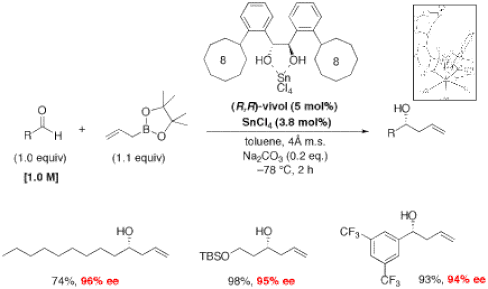
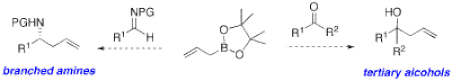
Organoboron compounds are extremely useful as stable reagents, intermediates, and catalysts in organic synthesis. Several applications of these compounds remain to be discovered, and our group is particularly interested in developing powerful asymmetric carbon-carbon bond forming methods based on boronic acids and their esters. The ability to selectively synthesize one of two enantiomeric forms of a molecule is very important in the pharmaceutical industry. The catalytic enantioselective allylboration of aldehydes recently optimized in our group (Figure 2)3 could potentially be extended to the preparation of chiral tertiary alcohols and amines using substrates like ketones and imines, respectively (Figure 3). This method makes use of diol-metal complexes as chiral Brønsted acids. The optimal diol we identified, Vivol, was nicknamed after Vivek Rauniyar, the graduate student from our group who discovered it.
2) Synthetic methodology and stereocontrol using organoboron chemistry
Organoboron compounds are extremely useful as stable reagents, intermediates, and catalysts in organic synthesis. Several applications of these compounds remain to be discovered, and our group is particularly interested in developing powerful asymmetric carbon-carbon bond forming methods based on boronic acids and their esters. The ability to selectively synthesize one of two enantiomeric forms of a molecule is very important in the pharmaceutical industry. The catalytic enantioselective allylboration of aldehydes recently optimized in our group (Figure 2)3 could potentially be extended to the preparation of chiral tertiary alcohols and amines using substrates like ketones and imines, respectively (Figure 3). This method makes use of diol-metal complexes as chiral Brønsted acids. The optimal diol we identified, Vivol, was nicknamed after Vivek Rauniyar, the graduate student from our group who discovered it.
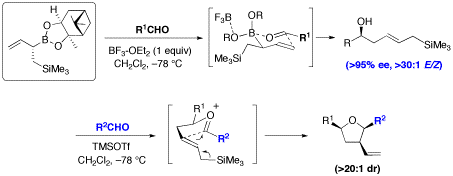 Figure 4. Example of application of B/Si double allylation reagent: synthesis of polysubstituted furans.
Figure 4. Example of application of B/Si double allylation reagent: synthesis of polysubstituted furans.
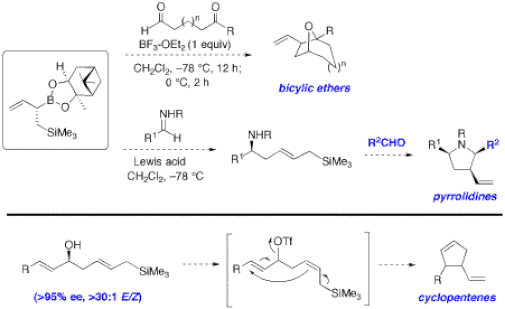
Recently, we disclosed a novel boron/silicon double allylation reagent that shows remarkable versatility in accessing skeletally different compound classes like propionate units, polysubstituted furans, vinylcyclopropanes, and oxabicycles (Figure 4).6 In the next few years, we will examine other applications such as the preparation of larger oxabicycles and polysubstituted pyrrolidines from an initial addition of the reagents to imines, as well as cyclopentenes from an initial addition to enals (Figure 5). We are currently planning applications of this novel class of reagents and other stereocontrolled organoboron based methodologies to the total synthesis of biologically and structurally interesting natural products (Figure 6). Lactone-containing terpenes display a wide range of biological activities and they are particularly interesting targets using a stereoselective tandem allylboration/lactonization optimized in our group. 16
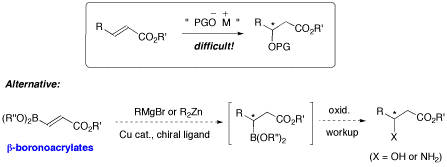 Figure 7. Asymmetric conjugate addition on b-boronoacrylates.
Figure 7. Asymmetric conjugate addition on b-boronoacrylates.
In the past few years our laboratory has been successful at exploiting the chemistry of substrates containing boronic esters as a temporary functional group. Along these lines, one future project will exploit the boronate group of 3-borono acrylates as a surrogate for hydroxy or amino groups in conjugate additions. There are very few effective method for stereocontrolled conjugate addition of hydroxy, alkoxy, or amine nucleophiles onto a,b-unsaturated carbonyl compounds (Figure 7). In contrast, tremendous progress has been made on catalytic asymmetric Michael-like additions with organozinc or organomagnesium reagents. To address the challenging problem of “heteroatom conjugate addition”, the known 3-boronoacrylates (and enones) could serve as excellent substrates in an indirect approach (Figure 7). The weak electron-withdrawing boronate substituent is expected to further activate the acrylate b-carbon, yet not enough to revert the regioselectivity. In this strategy, the resulting enantioenriched alkylboronate intermediates could be oxidized with retention of stereochemistry to afford important b-hydroxy or b-amino ester products. The resulting alkylboronates could also be employed in stereospecific cross-coupling reactions.
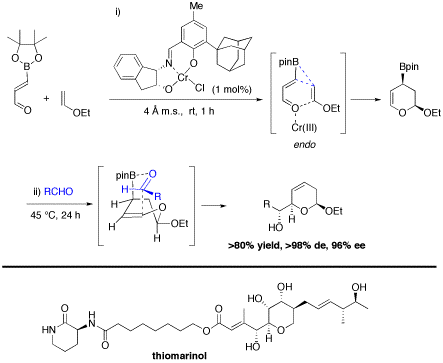 Figure 8. Three-component [4+2] cycloaddition/allylboration for the synthesis of a-hydroxyalkyl dihydropyrans, which was applied to a total synthesis of thiomarinol H.12
Figure 8. Three-component [4+2] cycloaddition/allylboration for the synthesis of a-hydroxyalkyl dihydropyrans, which was applied to a total synthesis of thiomarinol H.12
3) Multicomponent reactions and natural product synthesis
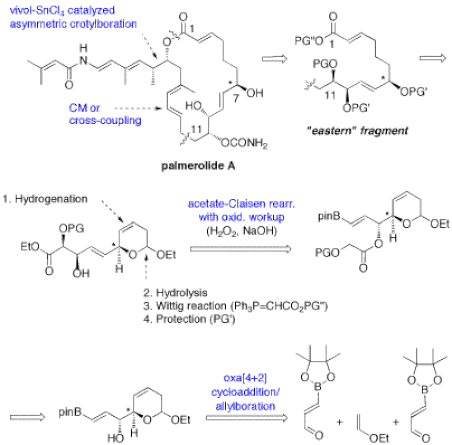
Multicomponent reactions are broadly defined as reactions that make use of three or more substrates simultaneously or sequentially without the need for changing the solvent. These atom-economical processes are particularly attractive in combinatorial chemistry, drug discovery, and even in natural product synthesis. In this context, our group has developed a tandem three-component [4+2] cycloaddition/allylboration strategy to assemble a-hydroxyalkyl piperidine derivatives parent to several alkaloids and azasugar analogues.5,14 A catalytic enantioselective variant has also been developed for making pyrans,4,15 and this multicomponent reaction was employed in the total synthesis of the potent antibiotic thiomarinol H (Figure 8).12 We are currently pursuing other applications of this powerful process along with other new reactions in the total synthesis of the novel anticancer agent palmerolide A (Figure 9). Palmerolide A was isolated from a tunicate in the Antarctic Ocean, and is a potent antimelanoma agent with great potential for the treatment of certain types of skin cancers. Following the completion of our total synthesis, we will design and test a series of analogues in collaboration with scientists at the University of Alberta Cross Cancer Institute.
Multicomponent reactions are broadly defined as reactions that make use of three or more substrates simultaneously or sequentially without the need for changing the solvent. These atom-economical processes are particularly attractive in combinatorial chemistry, drug discovery, and even in natural product synthesis. In this context, our group has developed a tandem three-component [4+2] cycloaddition/allylboration strategy to assemble a-hydroxyalkyl piperidine derivatives parent to several alkaloids and azasugar analogues.5,14 A catalytic enantioselective variant has also been developed for making pyrans,4,15 and this multicomponent reaction was employed in the total synthesis of the potent antibiotic thiomarinol H (Figure 8).12 We are currently pursuing other applications of this powerful process along with other new reactions in the total synthesis of the novel anticancer agent palmerolide A (Figure 9). Palmerolide A was isolated from a tunicate in the Antarctic Ocean, and is a potent antimelanoma agent with great potential for the treatment of certain types of skin cancers. Following the completion of our total synthesis, we will design and test a series of analogues in collaboration with scientists at the University of Alberta Cross Cancer Institute.
 Figure 10. Concept of bifunctional organocatalysis with boronic acids.
Figure 10. Concept of bifunctional organocatalysis with boronic acids.
4) Organocatalysis with boronic acids
The role of boronic acids as catalysts has long been neglected. These cool compounds have the unique capability of exchanging alcohols and carboxylic acids reversibly, and this property can be put to use in catalysis to achieve temporary activating and/or templating effects (Figure 10).
We have recently exploited this potential with the surprising and impressive discovery that ortho-haloboronic acids such as ortho-iodophenylboronic acid can catalyze direct amidation reactions between free carboxylic acids and amines at room temperature (Figure 11).7 This discovery has great practical implications because amides are one of the most important functional groups in Nature and in pharmaceuticals. In Nature, they are ubiquitous as the key units to the backbone of peptides and proteins. Unfortunately, traditional methods for the preparation of amides continues to challenge chemists and there are still no means to make amides in a simple, green and atom-economical fashion. In contrast, amidations catalyzed by ortho-iodophenylboronic acid do not create any waste, giving water as the only by-product, and the amide products can be isolated using just simple acid-base extractions.
The role of boronic acids as catalysts has long been neglected. These cool compounds have the unique capability of exchanging alcohols and carboxylic acids reversibly, and this property can be put to use in catalysis to achieve temporary activating and/or templating effects (Figure 10).
We have recently exploited this potential with the surprising and impressive discovery that ortho-haloboronic acids such as ortho-iodophenylboronic acid can catalyze direct amidation reactions between free carboxylic acids and amines at room temperature (Figure 11).7 This discovery has great practical implications because amides are one of the most important functional groups in Nature and in pharmaceuticals. In Nature, they are ubiquitous as the key units to the backbone of peptides and proteins. Unfortunately, traditional methods for the preparation of amides continues to challenge chemists and there are still no means to make amides in a simple, green and atom-economical fashion. In contrast, amidations catalyzed by ortho-iodophenylboronic acid do not create any waste, giving water as the only by-product, and the amide products can be isolated using just simple acid-base extractions.
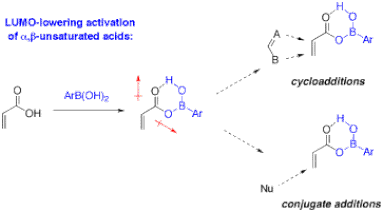 Figure 12. Proposed concept of organocatalytic activation of a,b-unsaturated carboxylic acids by boronic acids.
Figure 12. Proposed concept of organocatalytic activation of a,b-unsaturated carboxylic acids by boronic acids.
Ongoing work in this area of organocatalysis includes mechanistic studies, catalyst optimization, and applications to peptide synthesis and other reactions. For example, two other classes of reactions to be examined are the direct dipolar cycloadditions of unsaturated carboxylic acids as well as conjugate additions (Figure 12). The preparation of chiral arylboronic acids would lead to stereoselective applications such as the kinetic resolution of chiral racemic amines and carboxylic acids.
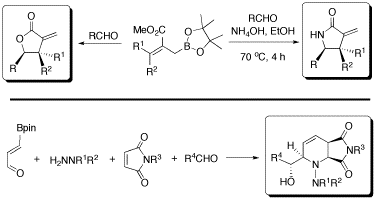 Figure 13. Libraries of bioactive compounds enabled by new methodologies.
Figure 13. Libraries of bioactive compounds enabled by new methodologies.
5) Combinatorial chemistry and chemical biology
Combinatorial chemistry is a relatively new field of research aimed at developing new methods and techniques to synthesize and evaluate large pools of compounds (called “libraries”) with ease and rapidity. As opposed to synthesizing and evaluating new compounds one-by-one, combinatorial libraries of new compounds can be made and screened as large mixtures containing as many as millions of compounds. Combinatorial chemistry is a powerful complementary technique in the drug search industry and other areas where the rational prediction of molecular properties can often be cumbersome and time consuming.
Our laboratory is involved in several areas of combinatorial chemistry, including solid-phase organic synthesis. A few years ago, we developed DEAM-polystyrene,8,9 the first solid support for immobilizing and derivatizing boronic acids. The DEAM-PS resin has become a successful commercial product used by several chemists worldwide. More recently, we have been interested in applying our new multicomponent reactions to synthesize parallel libraries of drug-like polycyclic molecules.17,18 Combinatorial libraries made of thousands of polysubstituted piperidines as well as hundreds of electrophilic lactones and lactams are synthesized and evaluated in high-throughput biological screening with different collaborators from campus, and elsewhere in Canada, US, and Europe (Figure 13). Many of these collaborations are developing into interesting joint projects in medicinal chemistry and chemical biology. For example, promising enzyme inhibitors were identified, which would not have been possible without the new chemistry developed in our laboratory. These libraries are assembled with state-of-the-art instrumentation for parallel synthesis such as a microwave reactor. When needed, we take advantage of a powerful strategy called “split-and-pool” solid-phase synthesis that makes it possible to synthesize thousands of compounds in less than a month!
Combinatorial chemistry is a relatively new field of research aimed at developing new methods and techniques to synthesize and evaluate large pools of compounds (called “libraries”) with ease and rapidity. As opposed to synthesizing and evaluating new compounds one-by-one, combinatorial libraries of new compounds can be made and screened as large mixtures containing as many as millions of compounds. Combinatorial chemistry is a powerful complementary technique in the drug search industry and other areas where the rational prediction of molecular properties can often be cumbersome and time consuming.
Our laboratory is involved in several areas of combinatorial chemistry, including solid-phase organic synthesis. A few years ago, we developed DEAM-polystyrene,8,9 the first solid support for immobilizing and derivatizing boronic acids. The DEAM-PS resin has become a successful commercial product used by several chemists worldwide. More recently, we have been interested in applying our new multicomponent reactions to synthesize parallel libraries of drug-like polycyclic molecules.17,18 Combinatorial libraries made of thousands of polysubstituted piperidines as well as hundreds of electrophilic lactones and lactams are synthesized and evaluated in high-throughput biological screening with different collaborators from campus, and elsewhere in Canada, US, and Europe (Figure 13). Many of these collaborations are developing into interesting joint projects in medicinal chemistry and chemical biology. For example, promising enzyme inhibitors were identified, which would not have been possible without the new chemistry developed in our laboratory. These libraries are assembled with state-of-the-art instrumentation for parallel synthesis such as a microwave reactor. When needed, we take advantage of a powerful strategy called “split-and-pool” solid-phase synthesis that makes it possible to synthesize thousands of compounds in less than a month!
6) Carbohydrate recognition in water
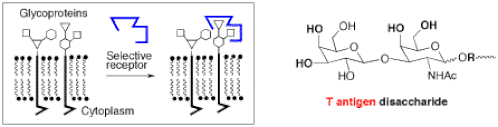
Cells are covered with complex sugar molecules (i.e., polysaccharides) displayed in the form of glycolipids and glycoproteins. This “sugar coating” of cells plays crucial roles in many physiological processes, disease states, and pathogenic threats of potential pandemic proportions like the anthrax bacteria, or the influenza virus (e.g., bird flu). The recognition and modification of the cell surface through their “glyco-signature” has yet to be solved and it has been deemed one of the most important problems in science. Although antibodies can be generated for binding to cell surface oligosaccharides, they are not always as selective as desired. Small receptor molecules could offer increased control and stability (Figure 14).
Cells are covered with complex sugar molecules (i.e., polysaccharides) displayed in the form of glycolipids and glycoproteins. This “sugar coating” of cells plays crucial roles in many physiological processes, disease states, and pathogenic threats of potential pandemic proportions like the anthrax bacteria, or the influenza virus (e.g., bird flu). The recognition and modification of the cell surface through their “glyco-signature” has yet to be solved and it has been deemed one of the most important problems in science. Although antibodies can be generated for binding to cell surface oligosaccharides, they are not always as selective as desired. Small receptor molecules could offer increased control and stability (Figure 14).
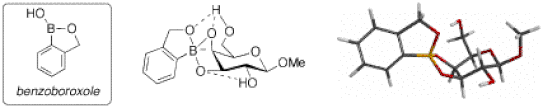
We address this challenge using boronic acids as key components of synthetic carbohydrate receptors. To this end, the ability of boronic acids to bind reversibly with alcohols is exploited. Indeed, diol units on sugars can bind to boronic acids even in water at physiological pH. Our group has recently discovered the first arylboronic acid capable of binding to the complex glycopyranosides (6-membered sugars) of the type found on cell surfaces. In this work, we showed that benzoboroxoles (Figure 15) can complex glycopyranosides under physiological conditions (i.e., in neutral water). 19,20
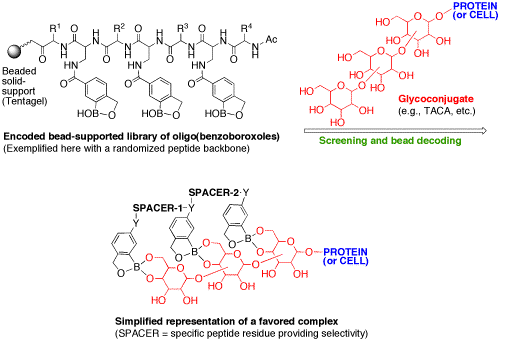
Although benzoborozole binds quite weakly to glycopyranosides, we exploit one of Nature’s clever principles: multivalency. More precisely, we make receptors with multiple units (i.e., oligomers) of boroxoles to increase the binding affinity. In our general approach, shown in Figure 16, we generate all possible combinations of peptide-based or polyamine-based oligomers of carboxy-functionalized benzoboroxoles.
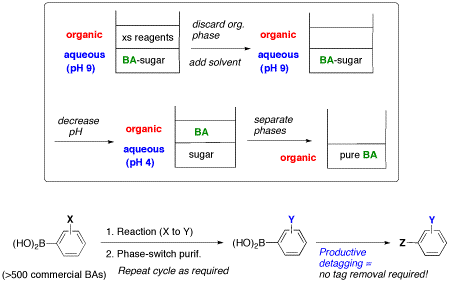 Figure 17. Phase-switch purification cycle with boronic acid tagged substrates (BA).
Figure 17. Phase-switch purification cycle with boronic acid tagged substrates (BA).
The library of oligoboroxoles was prepared using an Irori® system (radiofrequency encoding), and embodies a diversity of amino acid spacers that are expected to afford the binding selectivity. The resulting library was targeted against specific tumor-associatedcarbohydrate antigens (TACAs), such as the T-antigen (Figure 14), the sialyl Lewis X tetrasaccharide, or gangliosides. TACAs are found overexpressed on the surface of several types of cancerous cells, and their detection by oligoboroxoles could be important in cancer prevention and therapy and even in drug delivery. Our preliminary results are very promising, as shown by the discovery of a selective 20-micromolar receptor for the T-antigen disaccharide, an important cancer marker. In one application, an oligoboroxole receptor selective for the T-antigen (or other TACAs) will be labeled with a fluorescent probe and evaluated as a diagnostic tool for the detection of cancer. There is room for several new students in this project in the near future, as we face a number of important objectives such as the need for increasing throughput both in terms of library size and ease of screening, for example using microarray technology.
7) Green Chemistry – phase switch synthesis
Progress in chemical biology and medicinal chemistry relies extensively on the availability of large collections of novel small molecules of high purity. In response to these demands, new techniques and strategies are needed in order to accelerate and facilitate the synthesis and, especially, the purification of novel organic compounds. As a result of its wasteful use of solvents and silica, the purification of organic compounds tends to be environmentally costly. Phase-switching strategies are very attractive because they combine the respective advantages of solution-phase and solid-phase techniques. In phase-switch chemistry, reactions take place conveniently under homogeneous conditions, and product separation is facilitated by a phase separation such as precipitation, liquid-liquid partition, etc. Our group is currently optimizing a phase-switch system based on boronic acids as phase tags in a pH-driven water-organic biphasic system.21 Boronic acids are known to form water-soluble hydroxyboronate complexes with saccharides at elevated pH (~9). Using model arylboronic acids, we optimize phase separation between different organic solvents and water, under both basic and acidic pH and with diverse aqueous additives like mono- and polysaccharides or other polyols. As shown in Figure 17, a first partition at high pH allows unwanted organic-soluble materials to be discarded. Then, acidification is expected to return the boronic acid in its neutral stage and assure its extraction into the organic phase. A simple evaporation will deliver the pure product.
The resulting phase-switch system will allow the facile separation of boronic acid tagged products from impurities and side products. Boronic acid tags are ideal because of their robustness and compatibility with several reaction conditions. This new concept constitutes a significant advance over other phase-switch systems that require substrate attachment and cleavage steps. As depicted in Figure 17, a large number of commercially available boronic acids (>500) can serve as substrates, consequently, this phase-switch system can also circumvent the tag attachment step. Most importantly, boronic acids can be converted selectively into a wide variety of useful products to terminate the synthetic cycle concomitantly with the detagging operation. In the medium-term, multistep syntheses of relevant compounds, even small natural products, will be undertaken in order to test the concept and identify a broad repertoire of reaction conditions compatible with free boronic acids.
7) Green Chemistry – phase switch synthesis
Progress in chemical biology and medicinal chemistry relies extensively on the availability of large collections of novel small molecules of high purity. In response to these demands, new techniques and strategies are needed in order to accelerate and facilitate the synthesis and, especially, the purification of novel organic compounds. As a result of its wasteful use of solvents and silica, the purification of organic compounds tends to be environmentally costly. Phase-switching strategies are very attractive because they combine the respective advantages of solution-phase and solid-phase techniques. In phase-switch chemistry, reactions take place conveniently under homogeneous conditions, and product separation is facilitated by a phase separation such as precipitation, liquid-liquid partition, etc. Our group is currently optimizing a phase-switch system based on boronic acids as phase tags in a pH-driven water-organic biphasic system.21 Boronic acids are known to form water-soluble hydroxyboronate complexes with saccharides at elevated pH (~9). Using model arylboronic acids, we optimize phase separation between different organic solvents and water, under both basic and acidic pH and with diverse aqueous additives like mono- and polysaccharides or other polyols. As shown in Figure 17, a first partition at high pH allows unwanted organic-soluble materials to be discarded. Then, acidification is expected to return the boronic acid in its neutral stage and assure its extraction into the organic phase. A simple evaporation will deliver the pure product.
The resulting phase-switch system will allow the facile separation of boronic acid tagged products from impurities and side products. Boronic acid tags are ideal because of their robustness and compatibility with several reaction conditions. This new concept constitutes a significant advance over other phase-switch systems that require substrate attachment and cleavage steps. As depicted in Figure 17, a large number of commercially available boronic acids (>500) can serve as substrates, consequently, this phase-switch system can also circumvent the tag attachment step. Most importantly, boronic acids can be converted selectively into a wide variety of useful products to terminate the synthetic cycle concomitantly with the detagging operation. In the medium-term, multistep syntheses of relevant compounds, even small natural products, will be undertaken in order to test the concept and identify a broad repertoire of reaction conditions compatible with free boronic acids.
References
- D.G. Hall, Editor; Boronic Acids – Preparation and Applications in Organic Synthesis and Medicine; Wiley-VCH, 2005. [Book]
- D.G. Hall; Lewis and Brønsted Acid-Catalyzed Allylboration of Carbonyl Compounds: from Discovery to Mechanism and Applications; Synlett 2007, 1644-1655. [Publication 47]
- V. Rauniyar, H. Zhai, D.G. Hall; Catalytic Enantioselective Allyl- and Crotylboration of Aldehydes Using Chiral Diol-SnCl4Complexes. Optimization, Substrate Scope and Mechanism Investigations; J. Am. Chem. Soc. 2008, 130, 8481-8490. [Publication 55]
- X. Gao, D.G. Hall; 3-Boronoacrolein as an Exceptional Heterodiene in the Highly Enantio- and Diastereoselective Cr(III)-Catalyzed Three-Component [4+2]/Allylboration; J. Am. Chem. Soc. 2003, 125, 9308-9309. [Publication 22]
- B.B. Touré, H. Hoveyda, A. Ulaczyk Lesanko, J. Tailor, D.G. Hall; A Three-component Reaction for Diversity-Oriented Synthesis of Polysubstituted Piperidines: Solution and Solid-Phase Optimization of the First Tandem Aza[4+2]/Allylboration; Chemistry – A European Journal 2003, 9, 466-474. [Publication 17]
- F. Peng, D.G. Hall; Simple, Stable and Versatile Double-Allylation Reagents for the Preparation of Skeletally Diverse Compounds;J. Am. Chem. Soc. 2007, 129, 3070-3071. [Publication 46]
- R. Al-Zoubi, O. Marion, D.G. Hall; Direct and Waste-Free Amidations and Cycloadditions by Organocatalytic Activation of Carboxylic Acids at Room Temperature; Angewandte Chemie Int. Ed. 2008, 47, 2876-2879. [Selected a “Hot Paper” by the Editors][Publication 54]
- D.G. Hall, J. Tailor, M. Gravel; N,N-Diethanolaminomethyl Polystyrene: An Efficient Resin to Immobilize Boronic Acids; Angewandte Chemie Int. Ed. 1999, 38, 3064-3067. [Publication 2]
- M. Gravel, K.A. Thompson, M. Zak, C. Berube, D.G. Hall; Universal Solid-Phase Approach to the Immobilization, Derivatization, and Resin-to-Resin Transfer Reactions of Boronic Acids; J. Org. Chem. 2002, 67, 3-15. [This article was featured in the cover page graphics of Issue 1, 2002] [Publication 11]
- F. Wang, S. Manku, D.G. Hall; Solid Phase Syntheses of Polyamine Toxins HO-416b and PhTX-433. Use of an Efficient Polyamide Reduction Strategy That Facilitates Access to Branched Analogues; Organic Letters 2000, 2, 1581-1583. [Publication 4]
- S. H. Yu, M.J. Ferguson, R. McDonald, D.G. Hall; Brønsted Acid-Catalyzed Allylboration: Short and Stereodivergent Synthesis of All Four Eupomatilone Diastereomers with Crystallographic Assignments; J. Am. Chem. Soc. 2005, 127, 12808-12809. [Publication 36]
- X. Gao, D.G. Hall; Catalytic Asymmetric Synthesis of a Potent Thiomarinol Antibiotic, J. Am. Chem. Soc. 2005, 127, 1628-1629.[Publication 32]
- S. Manku, D.G. Hall; Synthesis, Decoding, and Preliminary Screening of a Bead-Supported Library of Triboronic Acid Receptors for Complex Oligosaccharides; Australian Journal of Chemistry 2007, 60, 824-828. [Publication 51]
- B.B. Touré, D.G. Hall; Three-Component Sequential Aza[4+2]/Allylboration/Retro-sulfinyl-ene Reaction: a New Stereocontrolled Entry to Palustrine Alkaloids and other 2,6-Disubstituted Piperidines; Angewandte Chemie Int. Ed. 2004, 43, 2001-2004. [Publication 26]
- X. Gao, D.G. Hall, M. Deligny, A. Favre, F. Carreaux, B. Carboni; Catalytic Enantioselective Three-Component Hetero-[4+2]Cycloaddition/Allylboration Approach to a-Hydroxyalkyl Pyrans: Scope, Limitations, Mechanistic Proposal; Chemistry European Journal 2006, 13, 3132-3142. [Classified a VIP paper by the journal] [Publication 39]
- T. Elford, S.H. Yu, Y. Arimura, D.G. Hall: Triflic Acid-Catalyzed Additions of 2-Alkoxycarbonyl Allylboronates to Aldehydes. Study of Scope and Mechanistic Investigation of the Reaction Stereochemistry; J. Org. Chem. 2007, 72, 1276-1284. [Publication 43]
- A. Ulaczyk-Lesanko, E. Pelletier, M. Lee, H. Prinz, H. Waldmann, D.G. Hall; Optimization of Three- and Four-Component Reactions for Polysubstituted Piperidines. Application to the Synthesis and Preliminary Biological Screening of a Prototype Library, Journal of Combinatorial Chemistry 2007, 9, 695-703. [Publication 50]
- T. Elford, A. Ulaczyk-Lesanko, G. De Pascale, G. Wright, D.H. Hall; Diversity-Oriented Synthesis and Preliminary Biological Screening of Highly Substituted Five-Membered Lactones and Lactams Originating from an Allylboration Reaction of Aldehydes and Imines, Journal of Combinatorial Chemistry 2009, 11, 155-168. [Publication 61]
- M. Dowlut, D.G. Hall; An Improved Class of Sugar-Binding Boronic Acids, Soluble and Capable of Complexing Glycosides in Neutral Water; J. Am. Chem. Soc. 2006, 128, 4226-4227. [Publication 41]
- M. Bérubé, M. Dowlut, D.G. Hall; Benzoboroxoles as Efficient Glycopyranoside-Binding Agents in Physiological Conditions: Structure and Selectivity of Complex Formation; J. Org. Chem. 2008, 73, 6471-6479. [Selected as a “Feature Article” and for Cover Page] [Publication 57]
- S. Mothana, S. Vanneste, N. Chahal, D.G. Hall; Phase-Switch Synthesis with Boronic Acids as Productive Tags; Journal of Combinatorial Chemistry 2007, 9, 193-196. [Publication 44]